- Stick to you reading and workbook schedule
- Group 3 and 4 meet tomorrow
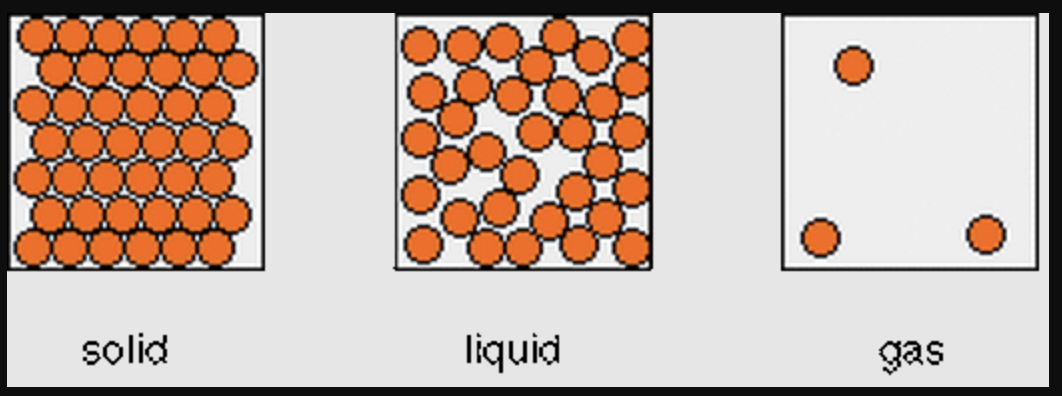
3.) SCIENCE TEST FRIDAY: Be sure to study the vocabulary and review the learning intentions.
4.) FRENCH:
- Pronunciation guide due Tomorrow
- Project Due (Monday)
- Make up five questions related to the science chemistry unit and post them here.
- Answer the five questions posted by one of your classmates.
- Reply original post giving feedback on the answers.
| | | |





 RSS Feed
RSS Feed
